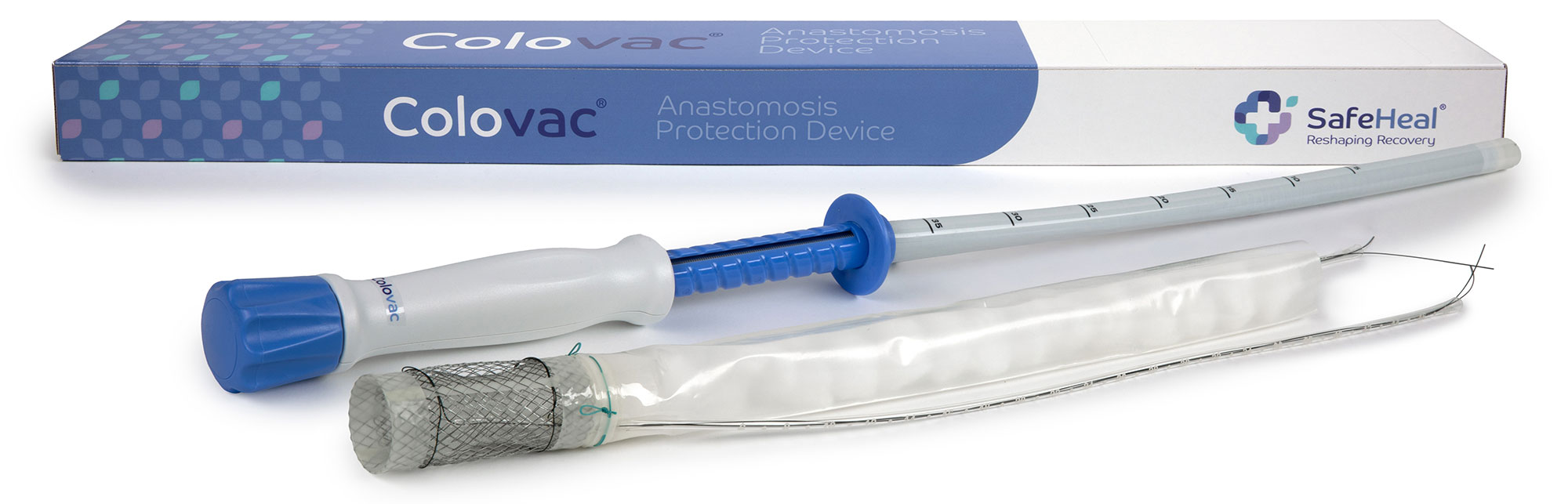
Established in 2015 and based in France, SafeHeal is a clinical stage medical device manufacturer. Specializing in the development of anastomosis protection devices for colorectal surgery, SafeHeal’s premier device, Colovac, was designed to eliminate the need for diverting ostomy in patients undergoing colectomy.
Feasibility and pilot clinical trials indicate that Colovac, by eliminating the need for a temporary stoma in the majority of low anterior cancer resection patients, may improve and expedite recovery from colorectal surgery. This is an especially important benefit for surgeons, clinicians, and postoperative care professionals, and for the community of colorectal cancer patients worldwide.
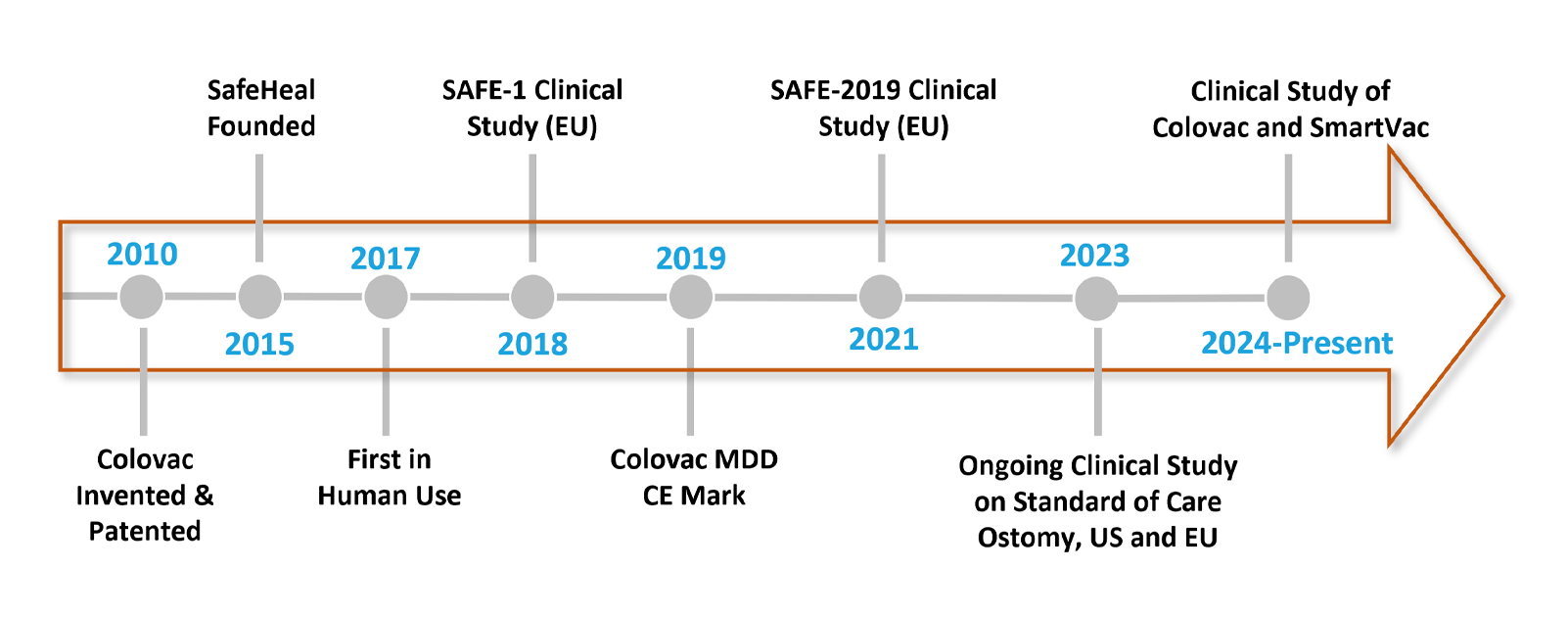
Milestones: Colovac in the Making
The SafeHeal Team

President & CEO
Chris has over 30 years of experience taking medical device startup companies from the clinical trial phase through worldwide commercialization and into acquisition. Chris has also held leadership positions at Boston Scientific and at Guidant.
Chris Richardson
President & CEO

VP, International
With more than 25 years of experience managing international business operations, Karl is a specialist in capital equipment and the development and production of medical devices and technology.
Karl Blohm
VP, International

VP, US operations
With 23 years in global medical device marketing and business development with large-cap corporations, Scott oversees SafeHeal’s U.S. operations toward commercialization as well as global expansion opportunities.
Scott DePierro
VP, US operations

VP, Regulatory Affairs and Quality
Marcos has over 25 years of experience in Regulatory/Clinical Affairs and Quality Assurance in support of the medical device industry including US and International, venture capital (VC) -funded start-ups, private, mid-sized and large public companies.
Marcos Velez-Duran
VP, Regulatory Affairs and Quality

VP, R&D, Manufacturing
Michael brings over 15 years of experience in biomedical engineering and project management, overseeing SafeHeal’s research and development and manufacturing.
Michael Augelli
VP, R&D, Manufacturing

VP, Clinical Affairs
Heather brings over 20 years of experience in leading global Clinical and Regulatory strategy for both large-cap public corporations and venture-funded startups. Heather leads SafeHeal’s clinical strategy and execution of rigorous clinical trials to support regulatory approval and commercial launch.
Heather Cronin
VP, Clinical Affairs

VP, Health Economics & Market Access
Jenny brings over 17 years of market access experience and a proven record of securing significant reimbursement and market access gains for innovative medical devices and procedures.
Jenny Gaffney
VP, Health Economics & Market Access
Board of Directors

- Andy Weiss
Mr. Weiss brings over 30 years experience in leadership positions of large, mid-size and early stage medical device companies, including GE, Marquette, and Medtronic. Since leading and overseeing the successful acquisitions of innovative medical device startups Vital Images, CoAxia, and ReCor Medical Systems, Mr. Weiss is currently a Director of May Health and Ziteo Medical.

- Anne Osdoit
Anne Osdoit runs MD Start Strategy, the in-house Medtech accelerator for Soffinova Partners. Anne is a Board member of both SafeHeal and AblaCare – two companies where she was also founding CEO. Anne is also the founding and current CEO of both Gradient Denervation Technologies and Moon Surgical.

- Antoine Papiernik
Antoine Papiernik is Chairman and Managing Partner at Sofinnova Partners, which he joined in 1997. Antoine has been an initial investor and active board member in numerous public and private companies, including SafeHeal. He has been selected twice for the Forbes Midas List, an annual ranking recognizing the world’s top venture capital investors.

- Jay Watkins
Jay Watkins is an operating executive with extensive experience founding and funding healthcare companies. He has served as a board member for both private and public medtech companies.

- Gilad Glick
Gilad is an experienced global medical device business leader and current Vice President, Venture Investments at Johnson & Johnson, leading a team of investors to initiate and manage equity investments that drive business innovation in the Pharma and MedTech sectors.

- Martin (Marty) Sands
Marty’s investment career spans about 40 years of deal experience ranging from seed stage, to late stage, to public markets, and corporate restructurings. He has spent a great deal of his time in Biotechnology, Life Sciences, Med Tech, and Healthcare. While he has been involved in a myriad of companies, he is currently serving as Co-Chairman of Optmed, Director of Osteal Therapeutics, Board Observer at Tempo Therapeutics, Board Member of North Hills Medical, and currently serves as co-managing member of more than a dozen SPVS.

- Eric Richman
Eric I. Richman began his career in biopharmaceutical venture capital at HealthCare Ventures and has held CEO roles at Tyrogenex, LabConnect, and PharmAthene, which was later acquired by Altimmune. He most recently served as a Venture Partner at Brace Pharma Capital and currently advises Broad Oak Capital, a life sciences private equity firm, and is also a Venture Partner at Allele Capital. Mr. Richman is the co-founder of Horizon Infusions and serves on the boards of Gain Therapeutics, MicroHealth, LabConnect, Polarity Bio and other organizations.

- Chris Richardson
Chris has over 30 years experience taking medical device startup companies from the clinical trial phase through worldwide commercialization and into acquisition. Chris has also held leadership positions at Boston Scientific and at Guidant.
Scientific Advisory Board

MD, The Mount Sinai Hospital, NY
Dr. Sylla is a Professor of Surgery and System Chief of the Division of Colon and Rectal Surgery at the Mount Sinai Health System in NYC, and the immediate past president of SAGES (Society of American Gastrointestinal and Endoscopic Surgeons 2023-2024). She is Board Certified in General and Colorectal Surgery, and completed dual fellowship training in Colorectal Surgery and Minimally Invasive and Bariatric Surgery. Her clinical expertise includes colorectal cancer, inflammatory bowel disease, benign colon and anorectal diseases. She is an internationally known expert in transanal endoscopic treatment of rectal cancer.
Dr. Patricia Sylla
MD, The Mount Sinai Hospital, NY

MD, PhD, Amsterdam UMC
In 2006 he was appointed as a Professor in Surgery. His field of interest in patient care and research is the (minimal invasive) treatment of complex IBD, rectal cancer and complication surgery. Bemelman is a EBSQ certified colorectal surgeon (Bologna 2005), secretary of the European Crohn and Colitis organization and president elect of the European Society of Coloproctology. He guided more than 30 PhD students, has more than 460 peer reviewed papers and a H index of 57. He is currently working in the Amsterdam University Medical Center location Meibergdreef with his colorectal co-workers Roel Hompes, Pieter Tanis and Christianne Buskens.
Pr. Willem Bemelman
MD, PhD, Amsterdam UMC

MD, PHD, SAINT ANTOINE HOSPITAL, PARIS
Prof. Lefevre is a digestive surgeon at the Saint-Antoine Hospital in Paris, one of the top academic departments of surgery in France. He is Professor of General surgery since 2015 at the Sorbonne University with a specialization in colorectal surgery. After one year as Gold Medal of Surgery of Paris, he completed his medical degree in 2008. He spent one year as Fellow in Oxford before his PhD in Physiology at the Pierre and Marie Curie University in 2012. His main research areas are colorectal cancers, especially the familial syndromes, and inflammatory bowel diseases.
Pr. Jérémie Lefevre
MD, PHD, SAINT ANTOINE HOSPITAL, PARIS
Awards & Recognition
The U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to investigate the use of Colovac®. Breakthrough Device designation expedites the review of innovative technologies that can improve the lives of people with life-threatening or irreversibly debilitating diseases or conditions.
SafeHeal is the first company created and incubated by MD Start, a medtech accelerator developing innovative medical devices based on inventions from physicians.
SafeHeal is laureate of the Worldwide Innovation Challenge 2015 edition (Concours Mondial de l’Innovation - Phases 1 and 2) and of the iLab grant program, which foster talent and bring out future champions of the French economy.
SafeHeal was elected as the most promising and innovative investor conference MedTech start-up at Biovision 2016 and, was a finalist for the global Medtech Innovator 2016 competition.
SafeHeal is a 2016 recipient of the Charles Foix grant which rewards innovations aimed at improving the quality of life of elderly people.
1 Colovac is an investigational device limited by Federal (United States) law to investigational use. In the European Union, Colovac is exclusively used for clinical investigations. The Colovac device has not yet received regulatory approval, therefore the potential risks and benefits are not yet established.
V10.1_06162025