Treatment of Colorectal Cancer
Colon cancer is the second most commonly occurring cancer in women, and the third most commonly occurring cancer in men, with over 1.9 million new cases worldwide in 2022. Of these 1.9 million cases of cancer in the colon, approximately 500,000 are located in the rectum, the lowest segment of the colon. The following information is specific to the treatment of rectal cancer.
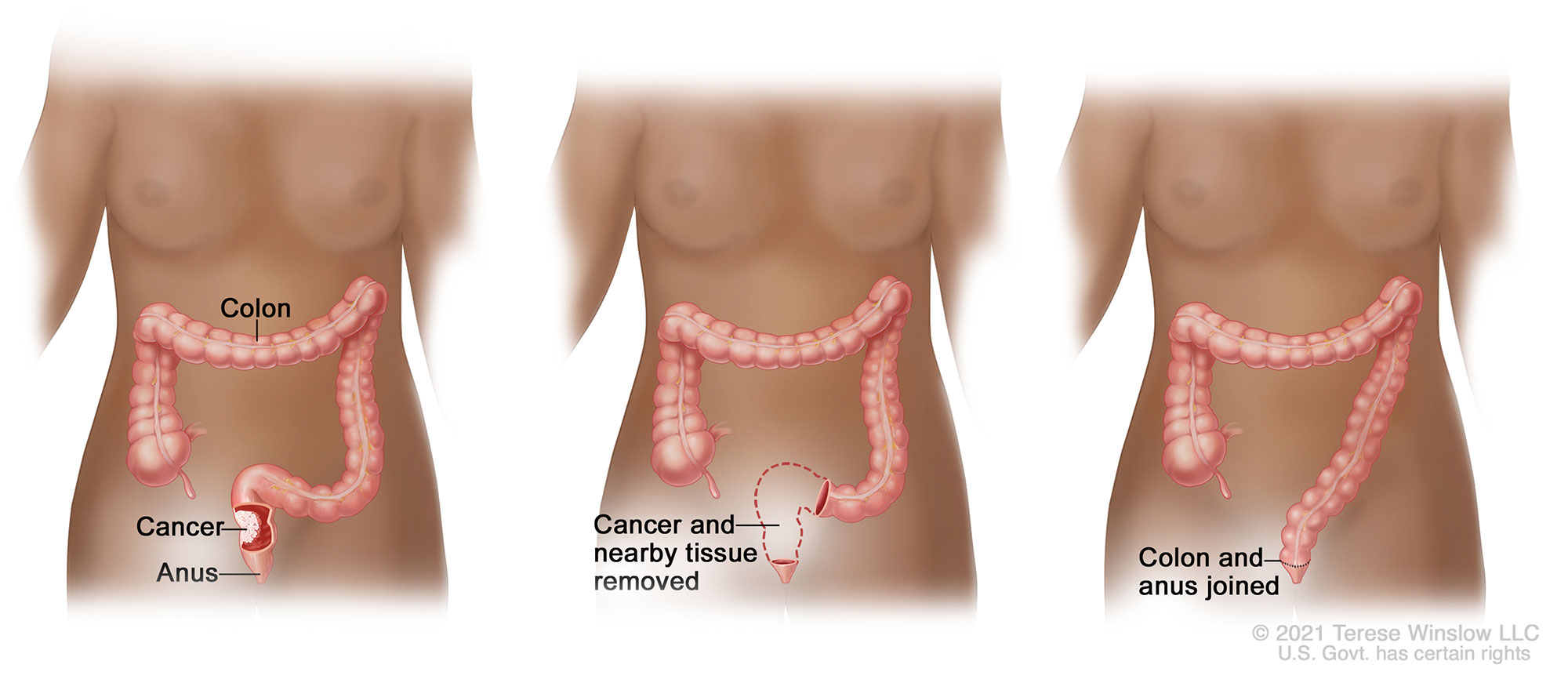
Treatment of rectal cancer most often involves radiation and chemotherapy followed by surgical removal of some or all of the rectum. The colon is then reconnected to the remaining rectum or anus with surgical staples or sutures; this connection is called an anastomosis.
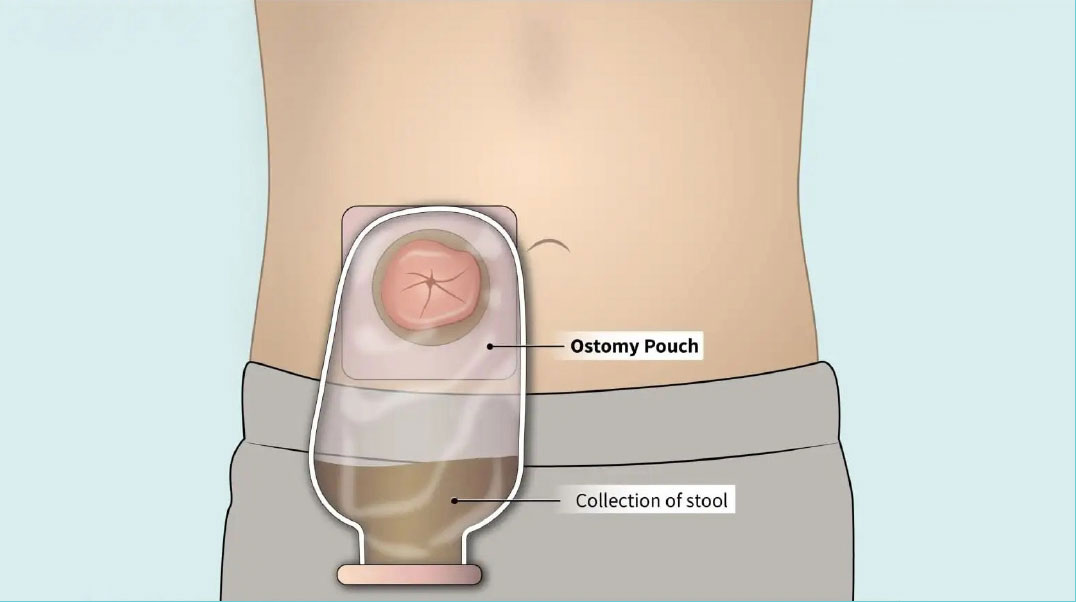
Because the risk of incomplete healing of the colon anastomosis is high, and the consequences could be dire, the surgeon may also create an ostomy (also called a stoma), which temporarily diverts the stool away from the healing anastomosis to the outside of the body and into an ostomy bag. Usually the ostomy is needed only until the rectum has healed, and then it can be reversed. If the entire rectum is removed, however, the ostomy may be permanent.
If the ostomy is intended to be temporary, the patient typically keeps the ostomy for approximately 2-6 months. The eventual reversal of the ostomy requires another operation, with a second hospital stay and recovery period.
Resection of the Rectum with Anastomosis
As with any surgery, complications can develop. While an ostomy procedure after rectal resection surgery is the current standard of care to provide protection to the healing anastomosis, specific risks are associated with the ostomy operation. The most common of these complications may include:
- Dehydration
- Irritation and inflammation of the skin around the ostomy
- A hernia at the site of the ostomy
Additionally, complications may develop from the secondary surgery 2-6 months later to reverse the ostomy.
In addition to physical complications, ostomy patients may experience an impact to their quality of life, due to:
- Social isolation
- Reduced physical activity and/or intimacy
- Extended recovery after cancer surgery
- Added expense of ostomy supplies
About Colovac1
Colovac is a colorectal anastomosis protection device intended to reduce the contact of fecal content at the anastomotic site following colorectal surgery. The device is placed at the time of the rectal resection surgery and is fully reversible. Colovac is designed to remain in place for 10 days, until the body’s natural healing and tissue repair processes are complete, after which it is removed during an endoscopic procedure similar to a colonoscopy, without the need for a second surgical intervention.
For the majority of patients, Colovac is expected to eliminate the need for a second surgery. This enables patients to resume normal activity after their cancer resection surgery without the stigma and complications associated with an ostomy procedure. Colovac is an investigational device, not currently available for sale. The safety and effectiveness/performance of Colovac is still being evaluated in clinical trials.

Rectal Cancer Treatment without Colovac
- Time to Full Recovery: 2-6 months
- 2 Surgeries, 2 Hospital Stays
- Risk of Complications
- 2 Surgeries Under Anesthesia
- Additional Incisions
- Potential Ostomy Complications
- Risk of Permanent Ostomy
- Quality of Life Due to Ostomy Bag
- Potential Social Isolation
- Impact to Physical Activity and Intimacy
- Added Expense of Ostomy Supplies
- Cost to Patient and Health Care System
- Multiple Surgeries and Hospital Stays
- Time to Full Recovery

Rectal Cancer Treatment with Colovac
- Time to Full Recovery: as little as 2 weeks
- Only 1 Surgery and Hospital Stay for the Majority of Patients
- Risk of Complications
- Single Surgery under Anesthesia
- No Ostomy Complications
- Patient Satisfaction
- No Ostomy Bag, No Lifestyle Changes for the Majority of Patients
- Faster Return to Normal Life
- Decreased Cost to Patient and Health Care System2
- Single Surgery and Hospital Stay
- Reduced Time to Full Recovery
1 Colovac is an investigational device limited by Federal (United States) law to investigational use. In the European Union, Colovac is exclusively used for clinical investigations. The Colovac device has not yet received regulatory approval, therefore the potential risks and benefits are not yet established.
2 Surgical Endoscopy, 14 August 2023, Cost associated with diverting ostomy after rectal cancer surgery: a transnational analysis.
V10.1_06162025